Ans 1) Process Flow diagram :
Gold recovery from the sulfide concentrate typically involves further refining, usually by roasting or oxidation of the wet air. Such pyrometallurgical or hydrometallurgical treatments themselves are typically accompanied by techniques of cyanidation and carbon adsorption for the final gold recovery. Hydrometallurgy involves the application of aqueous chemistry to recover metals from ores, concentrates and recycled or residual materials. This method is used to remove less electro-positive or less reactive metals such as gold or silver. Gold recovery chemicals such as lime, sodium carbonate, sodium hydroxide, and sulfuric acid are used for slurry pH adjustment; sodium sulfide is used for activation; water glass and phosphate are used for dispersal; flocculation and starch are used for flocculation.
The process flow diagram for the hydrometallurgical processing of the gold ore using the Carbon in Pulp ( CIP ) process has been shown here. The ROM ore is used as the feed stream. The gold bars are used as the product. The comminution, beneficiation and hydrometallurgical aspect the gold purification have been separated.
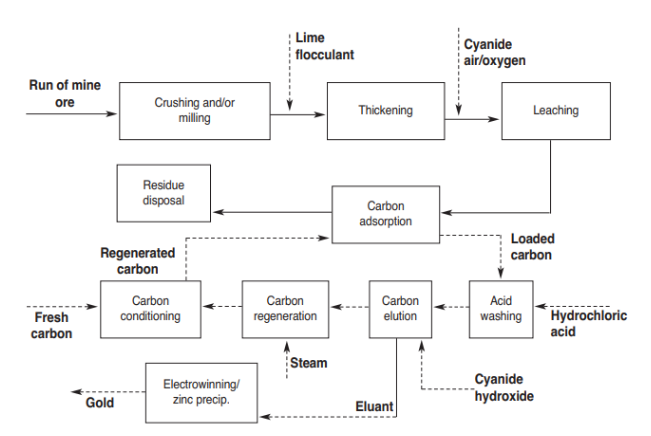
Figure 1
The Figure 1 shows the various steps. The run of mine ore is given to the crushing and/or milling stage. The next step is thickening which includes lime flocculant. The cyanide and air/oxygen are added for leaching process. Carbon adsorption is then done and residual disposal is done. Carbon adsorption gives loaded carbon for acid washing with hydrochloric acid ( Sole, 2018 ).
Then, carbon elution is done using cyanide hydroxide which generates eluant for electrowinning or zinc precipitation which generates gold. The carbon elution gives carbon regeneration using steam. Then, carbon conditioning is done using fresh carbon to give the regenerated carbon to the carbon adsorption stage ( Petrov, 2018 ).
The Carbon – in – pulp ( CIP ) comprises of the sequential leaching. After tis, the gold is adsorbed from the ore. In the CIP step, the pulp is made to flow from various agitated tanks. Here, the sodium cyanide and the oxygen are added for dissolving the gold in the solution. The gold yield is reduced by the attraction of the gold which was there for carbon in the activated form. In this method, leaching is done in the initial couple of tanks. Leaching is made to continue and the carbon adsorption takes place.
Ans 2) A stream table is produces for the process. The streams considered are which enter or exit the 4 PFDs. Data used in stream table is : Pso particles size, Water flowrate, solids flowrate and flowrate.
The design criteria is given by :
| Item | Value | Units |
| Total solids feature | 100 | Tonnes / hour |
| Solids feed Pso | 600 | Mm |
| Solids work index | 15 | kWh / tonne |
| Gold content | 3.5 | Grams /tonne |
| Silica content | 999996.5 | Grams / tonne |
| Weight (g) | Flow – Outlet 1 ( ml / CS ) | Flow – Outlet 2 ( ml / CS ) |
| 2001 | 1000 / 117 | 1000 / 134 |
| 18975 | 1000 / 116 | 1000 / 111 |
| 18864 | 1000 / 103 | 1000 / 145 |
| 189863 | 1000 / 95 | 1000 / 146 |
| Outlet flow ( ml / CS ) | Water content in concentrate recovered ( Weight % ) | Water content in cyanide recovered ( Weight % ) |
| 1000 / 45 | 9.6 | 9 |
| 1000 / 40 | 9.6 | 9 |
| 1000 / 48 | 9.6 | 9 |
| 1000 / 48 | 9.6 | 9 |
3. The half reaction for gold reduction is at the top of the electrochemical series, above the reaction for the disassociation of water.
The precipitation of gold from aqueous cyanide solutions is not thermodynamically spontaneous because gold is insoluble in water. Cyanide can help to stabilize gold in the solution which also needs oxygen for dissolving the gold. When gold mixes with dilute cyanide ( of sodium, potassium or calcium ) and air is bubbled then gold gets oxidised and forms aurocyanide complex ion ( Birloaga, 2014 ).
Gold shows a stable state with oxidation state of +3 in the electrolyte having the unit activity for the dissolved gold and the thiocyanate ions. If the concentration of the oxygen dissolved falls, then the equilibrium potential gets shifted to the negative side. Then, the +1 oxidation state becomes stable. If an oxidising agent is present, then gold is not stable thermodynamically. The electrowinning is required to precipitate gold out of cyanide solutions because the trace amounts of gold need to be recovered from the solutions.
Ans 4) Purpose of elution stage on a gold plant :
As we know that elution is the process of extracting any material from another material by the use of a solvent. The washing of loaded ions will exchange resin to remove the captured. It is the process of recovery of gold in the carbon in pulp process. The process requires the heating of caustic cyanide for many hours. Also sodium sulphite when used can affect the rate of reaction with full accuracy by four times ( Baba, 2014 ). The process is used to de absorb gold from activated carbon. Ethanol can also be used for the process. The solvent molecules called the eluate should travel down by the column. This phase is called as the stationary phase. This process displace by binding to the absorbent. After this phase the mobile column passes out of the column which is then collected form analysis. Since the process has a lot of safety concerns the process does not get accepted easily. In the process of gold extraction, the first column is called carbon column. Here the carbon absorbs gold form the solution that is provided ( Birloaga, 2013 ). The second step is the elution. In this process the carbon is released from the gold and both are separated.
References :
Baba, A. A., Ibrahim, L., Adekola, F. A., Bale, R. B., Ghosh, M. K., Sheik, A. R., … & Folorunsho, I. O. (2014). Hydrometallurgical processing of manganese ores: a review. Journal of minerals and materials characterization and engineering, 2(03), 230.
Birloaga, I., De Michelis, I., Ferella, F., Buzatu, M., & Vegliò, F. (2013). Study on the influence of various factors in the hydrometallurgical processing of waste printed circuit boards for copper and gold recovery. Waste management, 33(4), 935-941.
Birloaga, I., Coman, V., Kopacek, B., & Vegliò, F. (2014). An advanced study on the hydrometallurgical processing of waste computer printed circuit boards to extract their valuable content of metals. Waste Management, 34(12), 2581-2586.
Petrov, G. V., Fokina, S. B., Boduen, A. Y., Zotova, I. E., & Fidarov, B. F. (2018). Arsenic behavior in the autoclave-hydrometallurgical processing of refractory sulfide gold-platinum-bearing products. International Journal of Engineering and Technology (UAE), 7(2), 35-39.
Sole, K. C., Mooiman, M. B., & Hardwick, E. (2018). Ion exchange in hydrometallurgical processing: an overview and selected applications. Separation & Purification Reviews, 47(2), 159-178.