Abstract
Thermodynamics is a branch of science that deals with the condition of the body or surrounding in terms of temperatures and heat and their association with work, energy convection, radiation and other properties of matter. The calculations and the behavior of all these quantities is governed by two laws of four laws of thermodynamics .the first law of thermodynamic brings out the relationship of heat and the corresponding work done which is expressed in joules to the subjected body. It is as follows;
Where U is the change in internal energy, Q is the heat added and W is the work done by the system.
The second law of thermodynamic states that over a certain time the total entropy of an isolated system can never decrease and it is constant if and only if processes are irreversible.
Second law of the of thermodynamic is only applicable if the system has satisfied the first law of thermodynamics
Thermodynamic is applicable to the variety of topics both scientifically and in engineering field more so physical chemistry, chemical engineering and mechanical engineering among many more others. The earlier application of thermodynamics to mechanism such as heat engines was then quickly expanded to the study of chemical compounds and chemical reactions. The chemical thermodynamic filed deals or studies nature of the task of entropy in the process where it involves reaction of and has extract a lot of knowledge in the field where thermodynamics is involved.
Introduction
Thermodynamics is necessary for study in the technological world, it is essentially concerns with the study of body systems specifically internal motions for example the solids gases, light and liquids, it has a broad use in applications if the most ubiquitous part of the physics not in physics department. Thermodynamic is the integration of many subjects and it is of great significance part in the degree or diploma course
The study of thermodynamic has lately gains a lot of interest, many scientist want to study deep into science to the able to get a better understanding as well as yielding new important observation to scientific fields of theory and practical. Laws of thermodynamics especially the second law has been discovered to be of much use to engineers and the scientist who are interested in developing the technology. For example, the energy released due to the combustion of the fuel on the engine of a car was determined through thermodynamic experiments.
Thermodynamics is of much importance to our day to day life, we can utilize the knowledge through various ways some of these ways include
- The filling of petrol in the morning hours i.e.(8-9 AM), this is because during this hours there is lower atmospheric temperature as the density of petrol is high or more at evening hours i.e.(3-4 PM).
- Pressing of the clutch of the bike on the sloppy areas and when the bike is at its high speed i.e.(80-90 Km/h)
- Driving the bike at its lowest speed during the time of high temperatures this is because engine have lower thermal efficiency at such conditions.
People have started to get deeper understanding of the universe microscopically from classical calculation of thermodynamics
CALCULATIONS







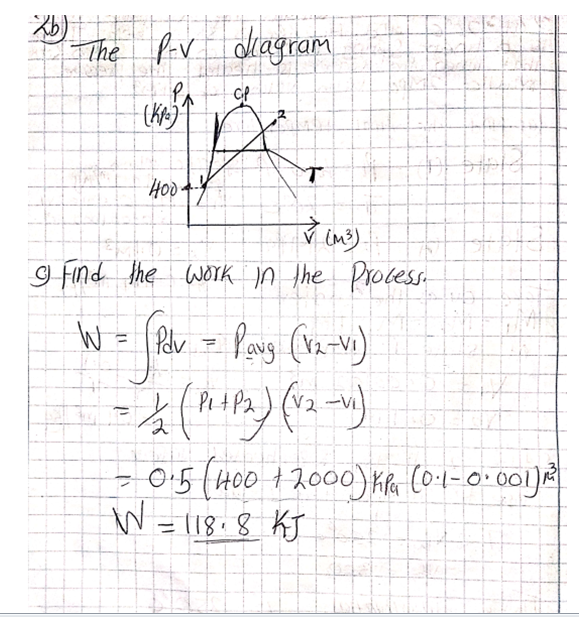
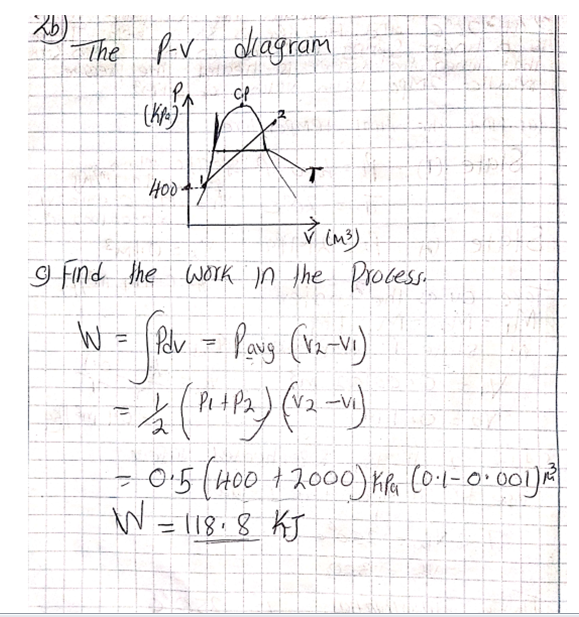
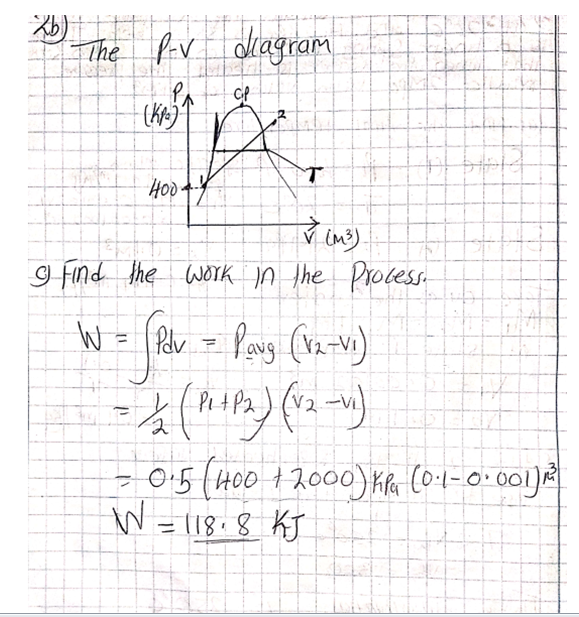




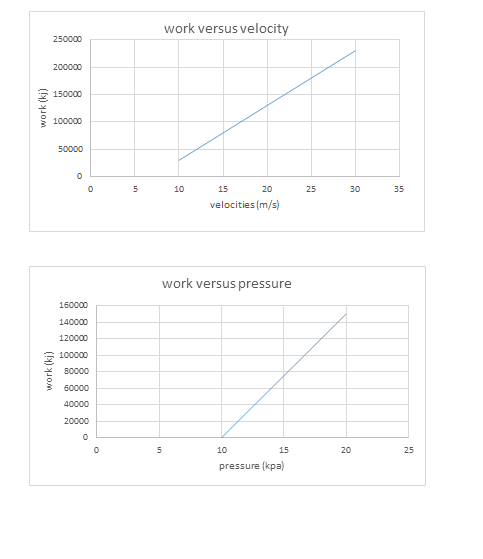
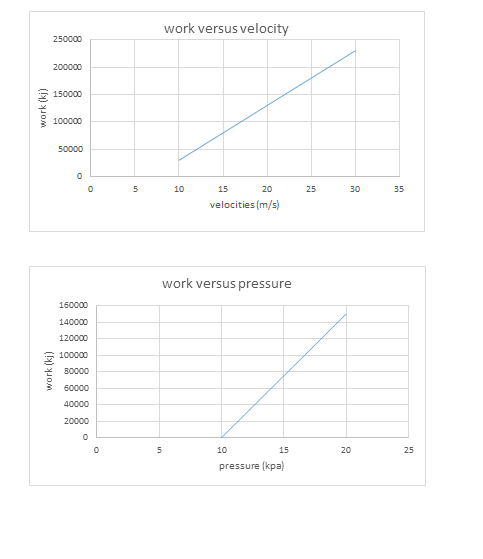
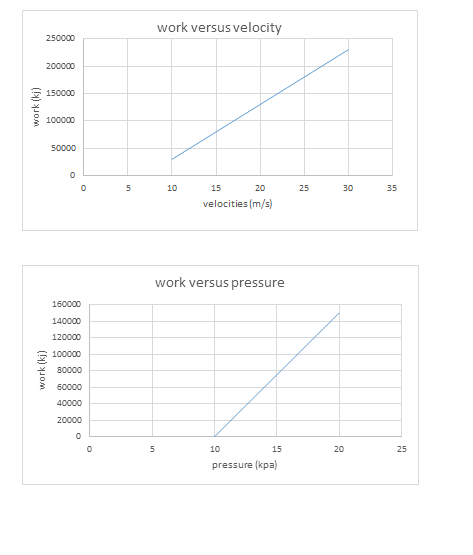

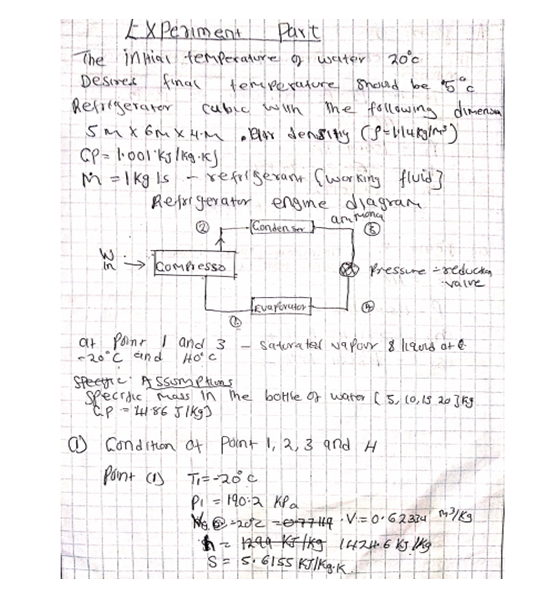
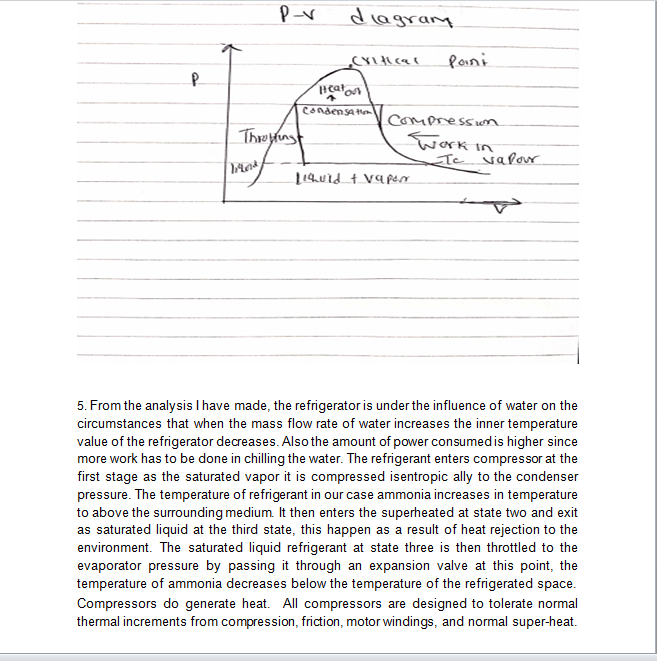
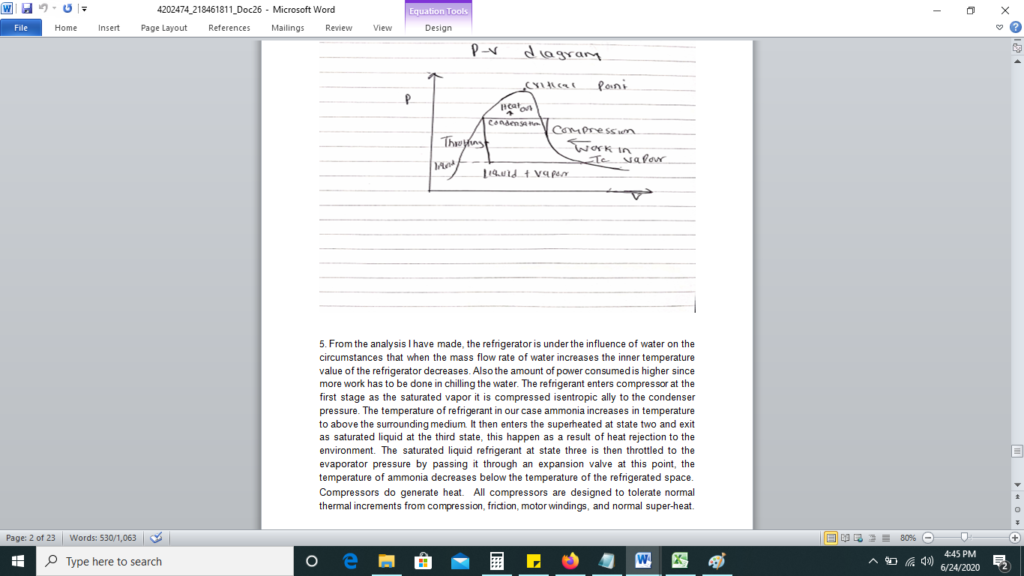
All this heat can be measured on a running system simply by taking the discharge line temperature about six inches or less from the compressor. If a discharge line temperature exceeds 250°F, the temperature inside the compressor at the discharge valve or valves is 300°F or more. At that high a temperature, oil and some refrigerants begin to break down. Carbon and sludge will form. Corrective action needs to be taken or the compressor will fail.
The Second Law of Thermodynamics essentially states that if a cold object is placed next to a hot object, the cold object will become warmer and the hot object will become cooler. The 2nd law speaks of entropy I think the zeroth law is more applicable. This principle applies perfectly on this study of refrigerator, since a refrigerator does not cool items by lowering their original temperatures; instead, an evaporating fluid called a refrigerant draws heat away, leaving the surrounding area much colder by working on principle of cooling through evaporation.
Conclusion
In conclusion on our review of refrigerator performance in 2008 (James and others 2008), more other surveys have been carried out around the world on the household storage that chilled the food. These studies, in general, continue to show remarkable similarities in householder attitudes, handling of chilled foods, and the performance of refrigerators around the world. It is still the case that, despite numerous recommendations on handling and storage temperatures, householder use and the performance of refrigerators appear to have remained remarkably unchanged throughout the world over the last 30 or so years. Many householders still do not follow recommended advice on the storage of chilled foods, fail to know or monitor the temperature of their refrigerator, and are storing chilled foods at higher‐than‐recommended temperatures.
References
William Thomson. (2012). Mathematical and Physical Papers. 1. London, Cambridge: C.J. Clay, M.A. & Son, Cambridge University Press. p. 232.
Cengel, Yunus A.; Boles, Michael A. (2005). Thermodynamics – an Engineering Approach. McGraw-Hill.
Duhem, P.M.M. (2009).Potential Thermodynamics Applications, Hermann, Paris.
Lewis, Gilbert N.; Randall, Merle (2014). Fundamentals of thermodynamics. McGraw-Hill Book. Guggenheim, E.A. (2013). Modern Thermodynamics by the Methods of J.W. Gibbs, Methuen, London.