A lab report: Dissolution test for Aspirin 75mg Tablets
Introduction
The importance of testing for the dissolution of drugs to the pharmaceutical industry is indisputable. Dissolution testing provides vital information to the producers of pharmaceutical compounds on the properties of the drug they produce. This information is useful in analysing consistency among the batches, regulation of the quality of the drug, establishing in vivo-in vitro correlation and predict the profiles to which the drugs will be released for human use (Matias et al., 2014, p. 3065). Among the most important aspects of a drug that dissolution test aim to establish is the bioavailability. Since solubility is entirely dependent on the solvent used, aprotic solvents such as dimethyl sulfoxide (lacking O, N and H atoms) are useful in investigating the solubility of drugs that are soluble in non-polar solvents.
Aspirin (acetylsalicylic acid) is a non-steroidal anti-inflammatory drug that is taken to relieve minor pain in the body and to control. It is an acidic (pH 3.5) drug with a crystalline appearance. It dissolves in organic solvents and has a molecular weight of 180.16. It slightly solubilizes in water and ethanol 30mg/ml and 50mg/ml respectively (Sievens-Figueroa et al., 2012, p. 1473). The carboxylic acid group and the ester are the chemical groups responsible for the biological activity of aspirin.
The dissolution of aspirin can be determined with the aid of an organic solvent. The procedure to conduct this involves the preparation of a stock solution of this drug by dissolving it in dimethyl sulfoxide (DMSO). Various concentrations can be derived from the stock solution by working the required calculations and making the appropriate dilutions. USP (United States Pharmacopeia) vessels maintained at 37oC temperature facilitate this process. The optical density values for the different concentrations prepared from the stock solution are measured. A linear regression curve for the optical density values is used to determine the percentage of the drug dissolved at a particular time.
In this experiment, the dissolution rate of Aspirin was determined by dissolving 75mg tablets in distilled water in a USP dissolution vessel. A linear regression curve was prepared from different concentrations of Aspirin made from stock solution dissolved in DMSO. The percentage of drug dissolved in water was determined from the linear regression curve.
Aims and Objectives
This experiment aimed to:
- Determine the rate of dissolution of Aspirin 75mg tablets in water.
- Determine the percentage of Aspirin dissolved in water after 6 minutes.
Principle
The temperature of the vessel used for testing the dissolution of Aspirin is maintained at 37oC to correspond to the body temperature of human beings for which its use is intended. A dissolution percentage of 70 after 10 minutes passes the criteria for dissolution testing for oral solid drugs (set up by the FDA).
Materials and methods
Materials
Dimethyl sulfoxide
Micropipettes (2uL, 10uL, 50uL, 100uL, 1mL, 2mL)
6 USP dissolution vessels (1-litre capacity)
6 Aspirin tablets (75 mg)
Stopwatch
Spectrophotometer and Cuvettes
Distilled water
Methods
6 Aspirin tablets (75mg) were placed into a USP vessel one at a time. A stock solution (10mM) of Aspirin was prepared by adding 1.8mg of the powdered form (pure aspirin) to 1ml of DMSO. From the stock solution (10mM), the following concentrations were prepared by adding the stated volume of Aspirin solution and toping up to 1ml using distilled water.
For example for the 50µl of stock solution was diluted with 950 µl of water to make a concentration of 0.5Mm.
Table of concentrations that were prepared
| Concentration (mM) | Stock solution (µl) | Amount of water added (µl) | Aspirin amount (µg/ml) |
| 0.5 | 50 | 950 | 90 |
| 0.3 | 30 | 970 | 54 |
| 0.2 | 20 | 980 | 36 |
| 0.1 | 10 | 990 | 18 |
| 0.05 | 5 | 995 | 9 |
| 0.02 | 2 | 998 | 3.6 |
Calculations on how the concentrations were prepared
Concentration of stock solution:
1.8mg was dissolved in 1.0ml of DMSO
Convert 1.8 mg to g = 1.8 mg/ 1000
= 0.0018g
Molarity = mass/ molecular mass
= 0.0018/ 180.1579 x 1000/1ml
= 0.00999M X 1000
= 10mM (rounded off)
Volume of the stock solution that is used to make the other concentrations
M1V1=M2V2
10mM x V1 = 0.3mM x 1ml
V1 = 0.3 x1/10
= 0.03 ml x 1000
= 30 µl
The same method for the rest to yield the amounts in the table = 50, 20, 10, 5 and 2 µl of the stock solution added.
Calculation of amount of Aspirin (µg/ml) in the concentrations prepared
10mM contains 1.8mg
1800µg 1000µl
50µl
1800µg x 50µl/1000µl = 90µg/ml
The same method was applied to get the amount of aspirin in the remaining concentrations as represented in the table.
The optical density (absorbance) of aspirin at these concentrations was determined using a spectrophotometer set at 270nm wavelength and recorded. The values obtained here were used to draw a linear regression curve. After 6 minutes the tablets that had been put in the USP vessel containing distilled water were removed and the percentage of the drug that had dissolved determined using the linear regression curve.
Results
Table of Absorbance of Aspirin at different concentrations
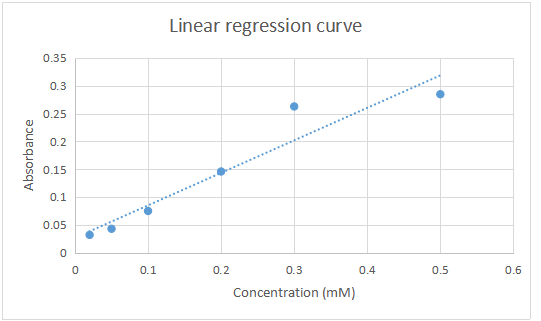
| Concentration (mM) | Absorbance at 270nm |
| 0.5 | 0.285 |
| 0.3 | 0.263 |
| 0.2 | 0.146 |
| 0.1 | 0.075 |
| 0.05 | 0.043 |
| 0.02 | 0.032 |
Linear regression curve for the absorbance against concentration
Absorbance of sample in the USP vessel after 6 minutes = 0.260
Calculation of percentage drug dissolved after 6 minutes:
- Molarity of the Aspirin 75mg dissolved in 900ml
Moles = mass/molecular mass
= 0.075g/180.1597 x 1000
= 0.416 millimoles
0.416 moles are in 900ml
For moles in 1000ml = 0.416 millimoles x 1000ml/900ml
= 0.462 millimoles/millilitre
= 0.462 mM
From the above table 90µg/ml is contained in 0.5mM of the solution
What about 0.462 mM
Concentration = 0.462 x 90/0.5
= 83.16 µg/ml
This represents 100% drug dissolution
- From the curve absorbance at 0.260 corresponds to a concentration of = 0.4mM
Therefore percentage drug dissolved
If 83.16 µg/ml is in 0.462mM
Amount of aspirin in 0.4mM = 0.4 x 83.16/0.462
= 72µg/ml
Percentage: 72/83.16 x 100 = 86.58%
- Value of x from the graph
Equation y = mx+c
Y = 0. 260
0.260 = 0.7265x + 0.0097
X= 0.260 – 0.0097/0.7265
= 0.3445
Discussion
The amount of Aspirin dissolved after 6 minutes was 72µg/ml corresponding to 86.58% of the amount that was dissolved in distilled water. From the table, the amount of Aspirin dissolved increases with an increase in concentration prepared. Aspirin having complete solubility in organic solvents has partial solubility in water. This solubility is contributed by the carboxylic acid group (COOH) that forms hydrogen bonds with the molecules of water (H20). The pH maintained at neutral contributes to the solubility. The pKa of Aspirin is 3.5 so at pH 7.0 (neutral) it is completely ionized resulting in maximum solubility. The dissolution test is done in water to correspond to the aqueous conditions in the stomach where this drug is absorbed (Anand and Patey, 2018, p. 243; Riley et al., 2012, p. 978).
After 6 minutes, 86.58% of Aspirin had dissolved. This was a very fast rate of dissolution and it passed the dissolution criteria for the drug. As a result, it is bioavailable at a high concentration just after 6 minutes. From the results, there were minimal errors and accuracy was optimized.
Conclusion
Aspirin 75mg tablets have a high dissolution rate of 86.58% in the water at neutral pH maintained at 37oC. After this time 72µg/ml out of 75µg/ml that was added had dissolved. Hydrogen bonds between water molecules and the carboxylic acid group of Aspirin are responsible for the dissolution, since, Aspirin has partial solubility in inorganic solvents and complete solubility in organic solvents such as DMSO. Testing for dissolution of Aspirin is important to pharmaceutical companies to determine the bioavailability and hence the efficiency by which it will exert biological action. However the dissolution rate in a non-biological system such as the USP vessels will not be identical to dissolution in a biological system such as the human body because many factors are responsible for the dissolution rate.
References
Anand, A. and Patey, G.N. (2018). Molecular dynamics simulation of aspirin dissolution. Journal of Molecular Liquids, 270, pp.243-250.
Matias, R., Ribeiro, P.R.S., Sarraguça, M.C. and Lopes, J.A. (2014). A UV spectrophotometric method for the determination of folic acid in pharmaceutical tablets and dissolution tests. Analytical Methods, 6(9), pp.3065-3071.
Riley, T., Christopher, D., Arp, J., Casazza, A., Colombani, A., Cooper, A., Dey, M., Maas, J., Mitchell, J., Reiners, M. and Sigari, N. (2012). Challenges with developing in vitro dissolution tests for orally inhaled products (OIPs). AAPS PharmSciTech, 13(3), pp.978-989.
Sievens-Figueroa, L., Pandya, N., Bhakay, A., Keyvan, G., Michniak-Kohn, B., Bilgili, E. and Davé, R.N. (2012). Using USP I and USP IV for discriminating dissolution rates of nano-and microparticle-loaded pharmaceutical strip-films. Aaps Pharmscitech, 13(4), pp.1473-1482.